lv reversible vs unreversible | reversible vs irreversible gas lv reversible vs unreversible We distinguish between two kinds of irreversible processes. A process that . From single-shade restorations to highly esthetic cases, you'll love the simplicity of TPH Spectra ® ST. Five universal CLOUD™ shades cover the full VITA ® range. Start with A2 shade for posterior and most common cases. Easier and faster shade selection with less inventory to maintain.
0 · reversible vs irreversible work
1 · reversible vs irreversible volume
2 · reversible vs irreversible process
3 · reversible vs irreversible pressure
4 · reversible vs irreversible gas
5 · reversible vs irreversible
6 · irreversible process vs reversible state
7 · example of reversible and irreversible process
Latvijas izlase ļoti labi zina, kādu basketbolu spēlē nākamā pretiniece Spānija, un tā būs spēle uz kļūdām, saka Rodions Kurucs #basketbols #delfilv. Delfi.lv
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we . A reversible process is one in which both the system and its environment can return to exactly the states they were in by following the . We distinguish between two kinds of irreversible processes. A process that .
reversible vs irreversible work
reversible vs irreversible volume
We distinguish between two kinds of irreversible processes. A process that .A reversible process implies there is no entropy generated as the system moves between two .
For reversible processes (the most efficient processes possible), the net change in entropy in .
A reversible process is truly an ideal process that rarely happens. We can make certain .
In animal studies, rapid atrial pacing has been shown to decrease the left ventricular ejection .A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we have \({dw}^{rev}<{dw}^{irrev}\) and\(\ w^{rev}
A reversible process is one in which both the system and its environment can return to exactly the states they were in by following the reverse path. An irreversible process is one in which the system and its environment cannot return together to exactly the states that they were in.
We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process. We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process.A reversible process implies there is no entropy generated as the system moves between two states (Sgen = 0 S g e n = 0). This does not mean that the entropy is constant. When the entropy remains constant (ΔS = 0 Δ S = 0), and the process is called isentropic.For reversible processes (the most efficient processes possible), the net change in entropy in the universe (system + surroundings) is zero. Phenomena that introduce irreversibility and inefficiency are: friction, heat transfer across finite temperature differences, free expansion, .
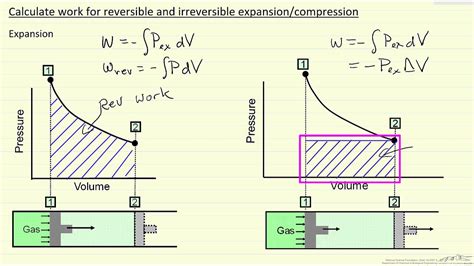
The diagram looks exactly how it should for a reversible process \to 1$. If the process is irreversible, on the other hand, the smooth solid line \to 1$ is deceptive, for it suggests that the system is passing through a sequence of equilibrium states in the process \to 1$. This is not the correct way to represent an irreversible process.A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference.In animal studies, rapid atrial pacing has been shown to decrease the left ventricular ejection fraction (LVEF) by roughly 52%. 3 Similarly, a 36% drop in cardiac index and 34% increase in cardiac size have been shown to occur with rapid right ventricular pacing. 4.
reversible vs irreversible process
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference.
From our definitions of reversible and irreversible pressure–volume work, we have \({dw}^{rev}<{dw}^{irrev}\) and\(\ w^{rev}
We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process.
We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process.A reversible process implies there is no entropy generated as the system moves between two states (Sgen = 0 S g e n = 0). This does not mean that the entropy is constant. When the entropy remains constant (ΔS = 0 Δ S = 0), and the process is called isentropic.For reversible processes (the most efficient processes possible), the net change in entropy in the universe (system + surroundings) is zero. Phenomena that introduce irreversibility and inefficiency are: friction, heat transfer across finite temperature differences, free expansion, . The diagram looks exactly how it should for a reversible process \to 1$. If the process is irreversible, on the other hand, the smooth solid line \to 1$ is deceptive, for it suggests that the system is passing through a sequence of equilibrium states in the process \to 1$. This is not the correct way to represent an irreversible process.
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference.
Depresija ir psihiska saslimšana, kas ietekmē emocijas, domas, uzvedību un ķermeņa fiziskās reakcijas. Tā izteikti negatīvi ietekmē spēju strādāt, mācīties, rūpēties par sevi vai citiem. Ikviens kādā dzīves brīdī var justies noskumis vai nelaimīgs, taču tas ne vienmēr nozīmē, ka sākusies depresija, definē SPKC.
lv reversible vs unreversible|reversible vs irreversible gas